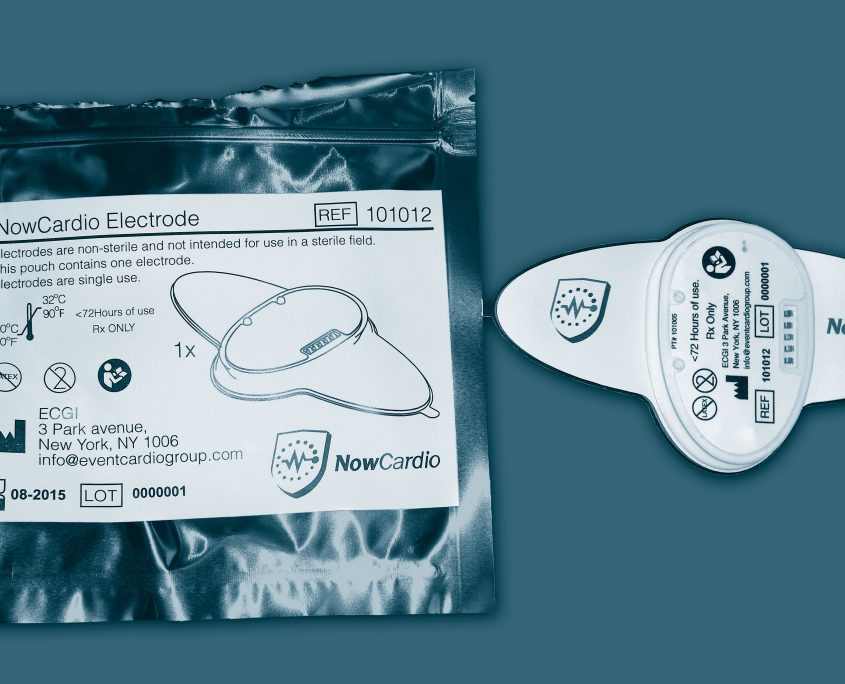
Maximize your medical device investment by taking advantage of the Contex Edge
CONTEX is an ISO 13485 certified company and offers to its customers, expert industry knowledge and engineering services to help deliver medical devices to Canadian and global markets with the assurance of “on-time” and “on-budget” delivery.
Our ‘concept -to- market’ approach focuses on providing both turn-key and contract engineering services to assist companies in avoiding costly mistakes and to help deliver medical devices that are safe, reliable, and profitable. Our team of qualified engineers and industry professionals will ensure that your Class I- I-III medical device development life–cycle is as stress-free as possible while assisting you with crucial regulatory submissions.